Through the efforts of the Global Myeloma Action Network (GMAN), these key points on the European (EU) drug approval and reimbursement policy were presented by Mait Raava from the Estonian Myeloma Society, during the GMAN meeting held in Copenhagen on June 11, 2016.
The presentation discusses the lack of a standardized cost-benefit formula across the EU, the impact of this on multiple myeloma (MM) patients, and GMAN's proposed solution and vision for the future.
To know more about European regulatory system for medicines, view the complete publication here.
- No standardized cost-benefit formula exists across the EU
- The Estonian reimbursement committee (Estonian Health Foundation) initially refused to approve bortezomib in 2013 as a frontline therapy for patients who were transplantation eligible but did so after the European Medicine Agency approved bortezomib (based on HOVON-65 results) in the same year.
- The Estonian reimbursement committee refused to approve bortezomib in first line for patients who are ineligible for transplantation in 2015, declaring that bortezomib and thalidomide results are NOT comparable.
2005 – 2009: the median OS in Estonia was 2.5 years*
2006-2010: the median OS at Mayo Clinic was 6.1 years**
- “The current results confirm continued survival improvement in MM and highlight the impact of initial therapy with novel agents.”
- “The improved survival is benefiting older patients and that early mortality in this disease has reduced considerably.”
* Innos K, Aareleid T. (2013). Vähielulemus Eestis 2005 – 2009. Eesti Arst, 92(8), 437-442.
** Kumar, S. K. et al. (2014). Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia, 28(5), 1122-8.
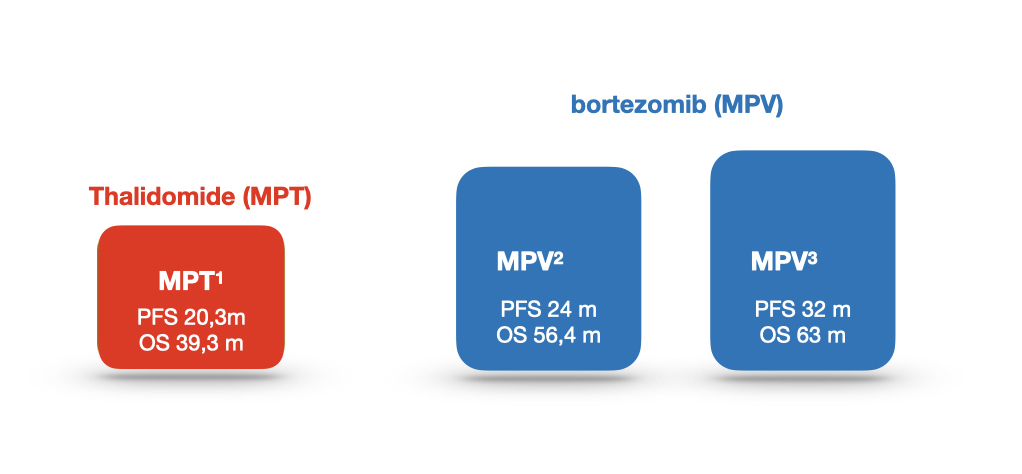
Multiple myeloma treatment schemes in the 1.
Line in elderly (1)
Multiple myeloma treatment schemes in the 1.
Line in elderly (2)
The retrospective case-matched study4
- If the European Medicine Agency approves a new medicine, it is not available in all European Union countries because each country decides differently about cost-benefit, using different formulas and information to determine approval.
- The assessment criteria used by each country is unclear and the data input for each country’s formula may or may not be based on scientific research.
- This creates a patchwork of approvals across the EU and makes predicting the chance of success for a particular drugs approval difficult.
- An assessment of a drug’s effectiveness from a single, scientific source that would be used as a universal measure across all EU countries.
- A standard formula for determining cost-to-benefit for reimbursement, instead of the individual and varied formulas currently used.
- Because of the availability of new drugs, myeloma patients in Eastern-European countries survive long in established European countries where new drugs are already available.
- If the cost-benefit analysis is reliable and valid it is possible to achieve acceptable pricing from pharmaceutical companies and the new drug will be accessible.
- Currently, the situation exists where national reimbursement committees reject the proposals based on confused arguments (e.g., because of “incomparable” studies etc.) and the price negotiation suffers from poor information.
- Collect data of available first line treatments for transplantation eligible and ineligible patients, and overall survival rates in each European country.
- Work with one another and our industry partners to engage politicians who have the authority to make changes happen and present them with our findings so that they support our position.
- Once support has been established, create a decisive action plan that educates the public and policymakers on the issue to ensure legislation is enacted.
European Medicine Agency approves the new drug only if:
- Drug’s effectiveness is proven by a common scientific body whose evaluation would be used as a benchmark across all EU countries.
- A standard formula is used for determining cost-to-benefit for reimbursement, instead of the individual and varied formulas currently used.
- Pharmaceutical company declares that it makes drug available in every EU country with the incremental cost (ICER) per QALY that doesn’t exceed per capita two GDP in each country.
1 Fayers PM, Palumbo A, Hulin C, Waage A, Wijermans P, Beksaç M, et al; Nordic Myeloma Study Group; Italian Multiple Myeloma Network; Turkish Myeloma Study Group; Hemato-Oncologie voor Volwassenen Nederland; Intergroupe Francophone du Myélome; European Myeloma Network. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011 Aug 4;118(5):1239-47.
2 San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma.J Clin Oncol. 2013 Feb 1;31(4):448-55.
3 Mateos MV, Oriol A, Martínez-López J, Teruel AI, López de la Guía A, López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. 2014 Sep 18;124(12):1887-93.
4 Morabito F, Bringhen S, Larocca A et al. Bortezomib, melphalan, prednisone (VMP) versus melphalan, prednisone, thalidomide (MPT) in elderly newly diagnosed multiple myeloma tients: A retrospective case-matched study. Am J of Hematol. 2014 Apr; 89[4]: 355-362.





![4 Morabito F, Bringhen S, Larocca A et al. Bortezomib, melphalan, prednisone (VMP) versus melphalan, prednisone, thalidomide (MPT) in elderly newly diagnosed multiple myeloma tients: A retrospective case-matched study. Am J of Hematol. 2014 Apr; 89[4]: 355-362.](/sites/default/files/GMAN%20visuals%20-%20EU%20Drug%20Policy.002%20%281%29.jpeg)